Mashhad University of Medical Sciences
Member of PERSIAN cohort network Vice Chancellery of Research
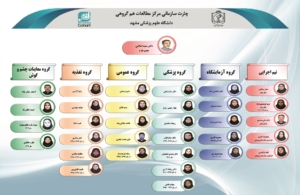
with cooperation of: Orthopedic Research Center , Lung Diseases Research Center , Nuclear Medicine Research Center , Endocrinology and Metabolism Research Center , Radiology Department of the MUMS
Principal Investigator: Dr. Saeid Eslami
– Department of Medical Informatics, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran. Pharmaceutical Research Center, Pharmaceutical Research Institute, Mashhad University of Medical Sciences, Mashhad, Iran. – Department of Medical Informatics, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran. – S.eslami.h@gmail.com EslamiS@mums.ac.ir – Tel/Fax: 98-5138827048, Cell: 989154403990
Approval date
11/02/2016
Starting date
10/21/2017
Goals
In addition to the scientific and executive objectives of the PERSIAN study, the center will move toward accomplishing the following goals: 1. To determine the incidence rate and natural history of Non-Communicable Disorders (NCDs) in health care providers, official staff, and professors. 2. To provide high quality database of ultrasound and 3D images for big data and machine learning researches. 3. To determine the prevalence of NCDs with regards to the bio-impedance, orthopedic, lung, cardiovascular, thyroid, liver, kidney, spleen, and biliary disorders. 4. Longitudinal study for medications
Study population
Professors and staff of the Mashhad University of Medical Sciences and Ferdowsi University of Mashhad.
Sampling method and sample size
All eligible volunteers who reserve a preferred date on the scheduling system are included. Mashhad University of Medical Sciences: 10000 participants in phase 1 Ferdowsi University of Mashhad: 2000 participants
Data Collection
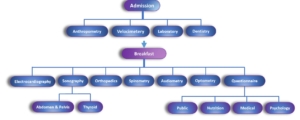
The baseline assessment includes a wide range of established and novel risk factors of NCDs obtaining by face-to-face interview or examination. Examinations include: Body size and composition test, Abdominopelvic and thyroid ultrasonography, Orthopedic evaluation, Pulse wave velocity test, Electrocardiography, Blood pressure measurement, Spirometery, Routine biochemical, cell count test, and hormones (TSH and TPSA),Smell and taste evaluation
Follow up Methods
Annual telephone interviews and repeated examinations at 5-year intervals are planned to update information on health status and its determinants.
Main Exposures
Demographic and socioeconomic characteristics, lifestyle pattern, fuel and pesticide exposures, occupational history and hazards, personal and familial medical history, medication profile, oral hygiene, reproduction history, dietary intake, and psychological conditions, Anthropometric measurements, biological variables, electrocardiogram, spirometery, and measurement of biochemical parameters NCDs and NCD-related morbidity and mortality including cardiovascular, bio-impedance, orthopedic, pulmonary, thyroid, liver, kidney, spleen, biliary, and psychological disorders
Outcomes
1. Death (confirmed cause of death)
2. Major NCDs including malignancies, cardiovascular diseases(myocardial infarction and other ischemic heart diseases, hypertension and heart failure), stroke, cerebrovascular accident, diabetes mellitus, pulmonary disease(Asthma, pneumoconiosis, COPD, chronic bronchitis and pulmonary emphysema), chronic renal failure leading to dialysis, hepatic fibrosis and cirrhosis, alzheimer and parkinson
3.trends in risk factors or protective factors including anthropometric, physiologic, nutritional, life style, environmental and occupational factors.
About The MUMS Persian Cohort